IMAGE
STANFORD CRITERIA
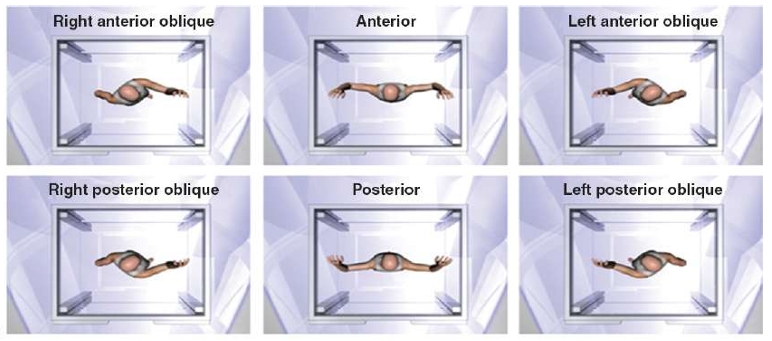
GANTRY POSITION
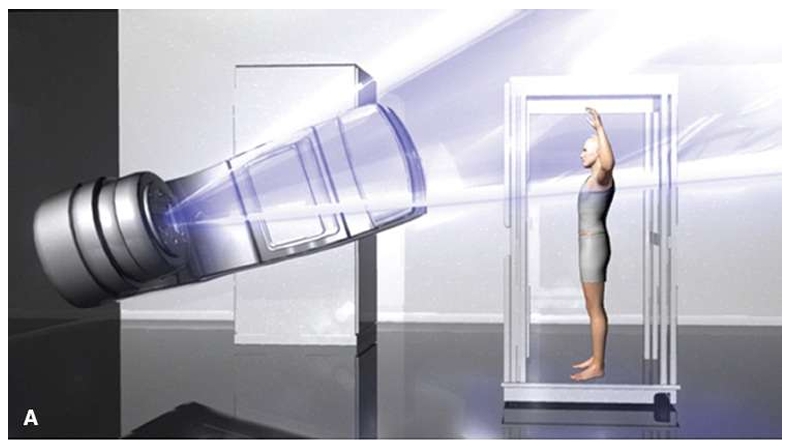
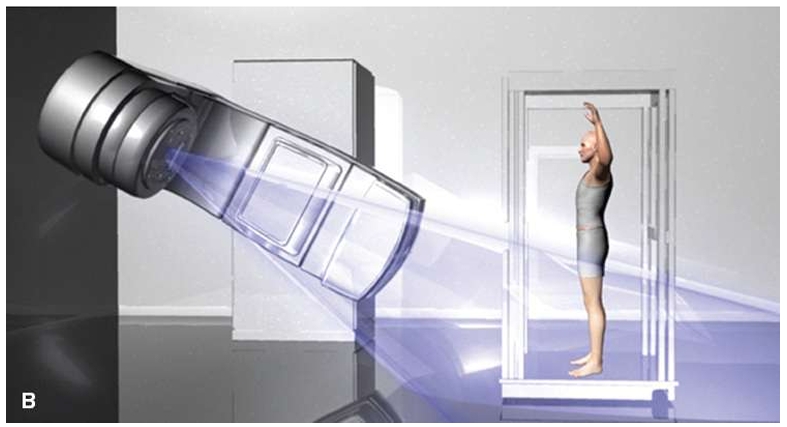
RadOnc Dr Revanth M Khandke
Total Skin Electron Beam Therapy (TSEBT) is a specialized radiation therapy technique used primarily for the treatment of cutaneous T-cell lymphomas, particularly mycosis fungoides.
Adjuvant PUVA:
Nitrogen Mustard:
Cycle Day 1 (Yale and Stanford Protocol):
Cycle Day 2:
Yale and Stanford Protocol:
| Depth | Dose Percentage |
|---|---|
| 1 mm | Maximum dose (100%) |
| 6 to 7 mm | 80% dose |
| 12.5 mm | 20% dose |
| Total Dose | Complete Response Rate |
|---|---|
| <10 Gy | 18% |
| 10 to 20 Gy | 55% |
| 20 to 25 Gy | 66% |
| 25 to 30 Gy | 75% |
| 30 to 36 Gy | 94% |
Important Consideration:
Lower doses yield impressive rates of overall response (defined as >50% reduction in cutaneous disease), overall survival, progression-free survival, and relapse-free survival rates. However:
Application of reduced-dose TSEBT (10 to 20 Gy) in combination with additional therapies may be a viable alternative to the current standard of 36 Gy.
Stanford/MD Anderson Trial:
The six treatment positions in TSEBT maximize unfolding of the skin and exposure of the skin surface to the incident electron beam, but certain areas remain obscured and require supplemental doses.
Target Dose: Minimum of 20 to 28 Gy at depth of approximately 4 mm
120-kV Superficial Photons:
Low-Energy Electrons (6 MeV):
Angled Electron Reflector Method:
Alternative Approach:
Other Areas That May Need Supplemental Dose:
The need for supplemental dose to such areas is based on the judgment of the radiation oncologist.
Patient Perception:
In a review of perceptions of MF therapy, patients overall considered TSEBT to be a more difficult treatment to endure compared to other treatments.
Important: Patients should be advised that symptoms such as pruritus and cutaneous erythema may be exacerbated during therapy.
| Side Effect | Description |
|---|---|
| Xerosis | Dry skin |
| Dry Desquamation | Peeling of skin |
| Extremity Edema | Swelling of arms/legs |
| Blister/Bullae Formation | Particularly over lower extremities |
| Alopecia | Hair loss from scalp, eyebrows, eyelashes, and body (Usually regrows, but may be lost) |
| Nail Changes | Nails may ultimately be lost but usually regrow |
| Hypohydrosis | Secondary to damage to sweat glands |
| Nasal Irritation | Dryness and irritation may result in nose bleeds |
| Gynecomastia | Rare occurrence |
Late/chronic side effects are minimal and include:
Clinicians should be vigilant for the possibility of active cutaneous infection during the course of therapy.
Areas most susceptible to TSEBT are blocked during certain cycles of treatment:


